In November 2018 the U.S. FDA and CDC advised consumers to avoid romaine lettuce, since it was not possible for consumers to distinguish the growing regions under investigation and those that were not implicated. As a result, many romaine suppliers agreed to include the growing region, or provenance, on consumer units. This questions-and-answer document, originally drafted by PMA and United Fresh in December 2018 and updated May 2019 by a cross-section of the industry, has been further updated based on learnings from the advisory issued in November 2019.
Mission: To provide members of the fresh produce supply chain and consumers easily understandable and accessible information about growing regions associated with romaine products.
Vision: This is an interim step until better traceback mechanisms are adopted across the supply chain.
This document addresses recommended provenance labeling for romaine-containing items that are packaged in consumers units, as well as cases that are used to ship romaine (in bulk or in processed forms). The recommendations are summarized in the table below, which is followed by a detailed Q&A that industry members should reference. This guidance is aligned with best practices for case labeling developed by a separate Produce Traceability Initiative workgroup.
The table below summarizes how provenance/region information can be communicated on a case and on a consumer unit. Please read the additional details on this webpage for more information and context.
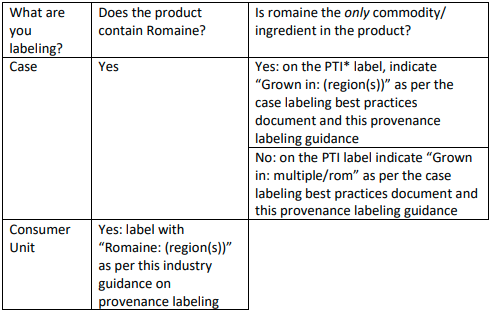
*PTI labels (Produce Traceability Initiative) is recommended for all produce. See questions 7&9 for more information on labeling cases, including when a PTI label is not applied, or is not used to communicate growing region.
- What products are covered by this effort? At this time, romaine lettuce including processed blends containing mature or baby romaine lettuce are covered.
- What should be labeled? The growing region should be indicated on consumer-level packages as recommended in this guidance. Cases/boxes containing romaine (only), whether processed or not, and including commodity romaine, should also bear this information on the PTI label (as per the Best Practices for Formatting Case Labels) or through an alternative means.
- When should this information be included on packages for consumers? Immediately and until further notice. This is an interim step until better traceback mechanisms are in place.
- Does the provenance labeling apply to other leafy greens? Not at this time.
- Is this a new regulation from FDA? No, the indication of growing region for romaine on consumer packages is voluntary but is encouraged by FDA. Although this is a voluntary effort, some retailers and foodservice operators are requiring it. Also, it there are outbreaks in the future associated with romaine, FDA and CDC may advise consumers that they should look for/ask for this information when purchasing or eating romaine to ensure that the available romaine is not from growing regions that could be associated with the outbreak. In addition, they are likely to advise consumers to avoid romaine products that lack growing region information.
- For consumer level packages, what terminology should be used (for both field packed and processed romaine)?
a. Romaine: (region(s)):
i. See Question 12 for recommended regions.
ii. See example package at the end of this document (Appendix A)
b. For indoor growers, the information in (a) should be preceded by the phrase: Indoor grown - For cases/cartons moving through the supply chain that contain only romaine (processed or not), what terminology should be used? If the shipper/processor uses PTI, then when a case only contains romaine, the PTI label should bear the harvest region(s) of romaine by printing "Grown in (region(s))" on the PTI label (note: the item name, in this case romaine, would also be printed as per the standardized PTI label).
a. See Question 12 for recommended regions
b. See example PTI label at the end of this document (Appendix B)
c. A separate workgroup organized under the Produce Traceability Initiative established recommendations for including romaine origin on PTI case labels. If the shipper/processor does not use PTI, an alternate mechanism (e.e., a sticker) should indicate the region(s) from which romaine was sourced. - What if the consumer unit product contains romaine from multiple growing regions? If product is commingled and contains romaine harvested from multiple regions, then an “and” statement should be included on the consumer unit or the regions should be separated by a comma; e.g. Romaine: (region), (region) or Romaine: (region) and (region). Labeling should be accurate and could include the use of an “and/or” declaration if romaine from multiple growing regions is in the product. However, if an advisory is issued consumers will likely be directed to avoid romaine that could have come from a region subject to the advisory. Therefore, the use of and/or should be as limited as possible.
- What if packages or cases contain romaine as well as other ingredients, including other leafy greens?
a. For consumer packages: the identification of the romaine ingredient should be indicated as noted in question 4. Growing regions for other ingredients are not recommended.
b. For cases:
i. Bearing a harmonized PTI label: A separate workgroup organized under the Produce Traceability Initiative established recommendations for including romaine origin on PTI case labels. For cases that contain, for example, salad blends consisting of multiple types of leafy greens or other non-romaine ingredients, do not indicate a growing region on the PTI label (since it would be unclear with ingredient was being referenced). Instead, indicate "Grown in: multiple/rom". In the event of an advisory, this information would signal that the item did not contain romaine, and serve as a cue for the recipient to open the box and check the package to determine the growing region for romaine. Additional information can be found in the Best Practice for Formatting Case Labels available from producetraceability.org.
ii. Without a harmonized PTI label: there is flexibility in how to indicate that a) the contents of the case contain romaine and b) the growing region(s). Some producers have retained terminology consistent with the consumer unit labeling (romaine: region(s)) either on a PTI label or other marking/sticker on the case. - I grow and/or ship bulk romaine for regional processing. Do I have to label anything? While voluntary, yes, it is recommended that growing region be indicated in the human readable space of the PTI label or elsewhere on the case (as discussed in question 7), so that the processor is able to identify growing region(s) on the finished product.
- What is the definition of “indoor grown” and how can it be labeled? It is a controlled environment for growing (not Hoop houses). We recommend that indoor growers follow the label standards and be labeled “Indoor grown” followed by the region, i.e. “Indoor Grown Romaine: (region)”
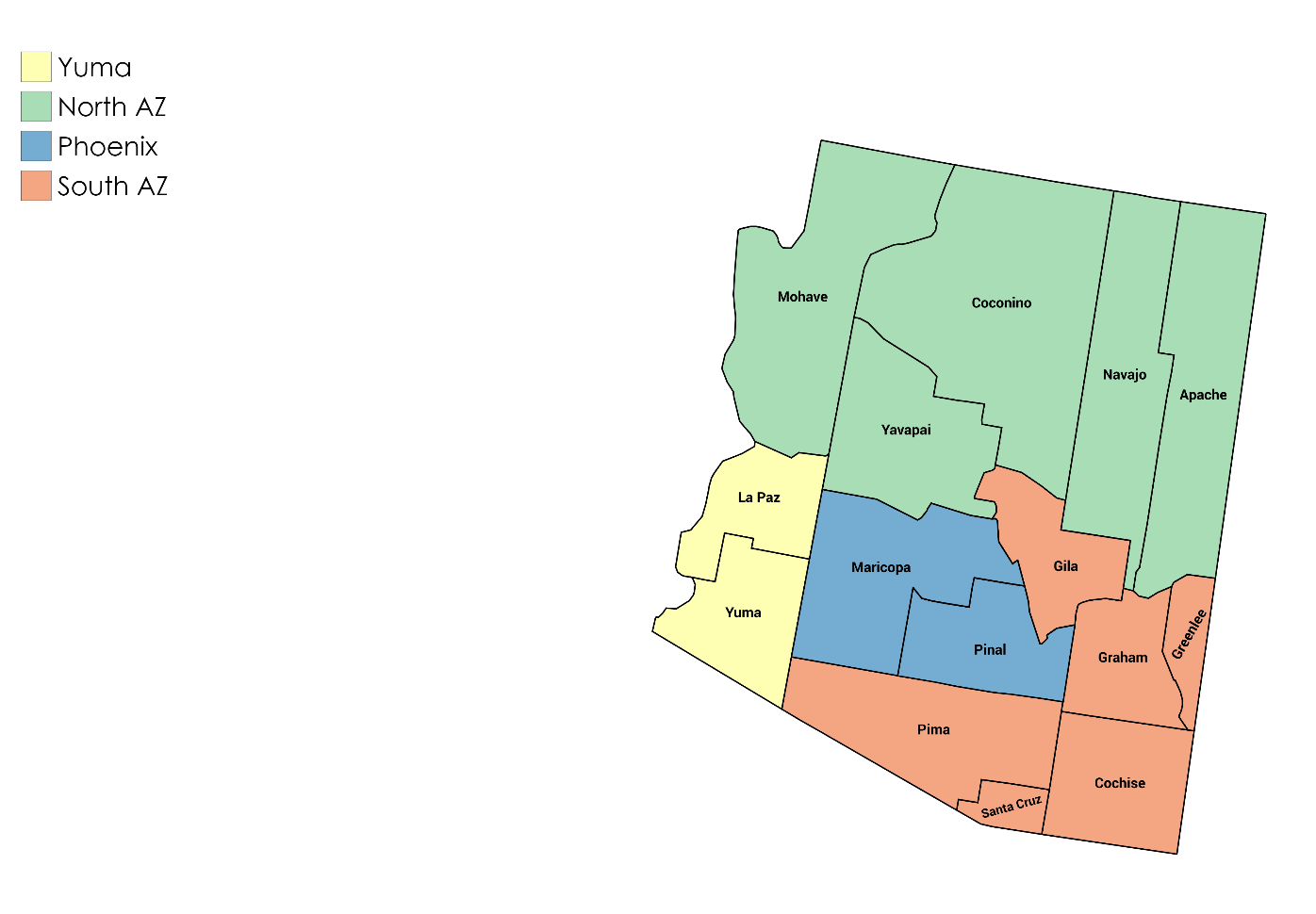
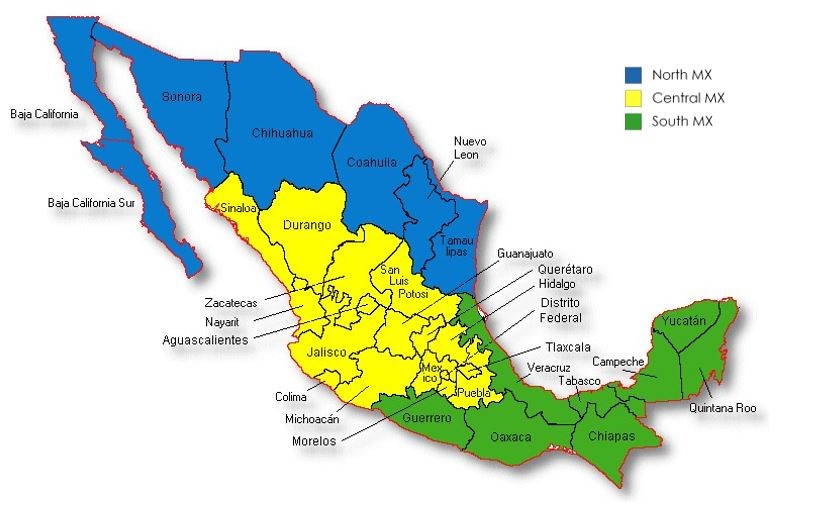
- How should growing regions be named? A workgroup within the 2019 romaine task force suggests that growing regions be communicated on the label using the designations and abbreviations listed below that are based on the boundaries indicated in the accompanying map. The regions, terminology, and abbreviations were reviewed in the spring of 2020 and no changes were recommended.
*If not listed, use the 2-letter state, province or territory abbreviation unless otherwise noted.Growing Region Abbreviation Yuma** Yuma Phoenix Phoenix Southern Arizona South AZ Northern Arizona North AZ Northern California North CA Salinas Salinas Santa Maria Santa Maria Southern California South CA Imperial Valley Imperial Vly Coachella Coachella Central Valley Central Vly Northern Mexico North MX Central Mexico Central MX Southern Mexico South MX
**Note: "Yuma" includes Bard and Winterhaven, CA.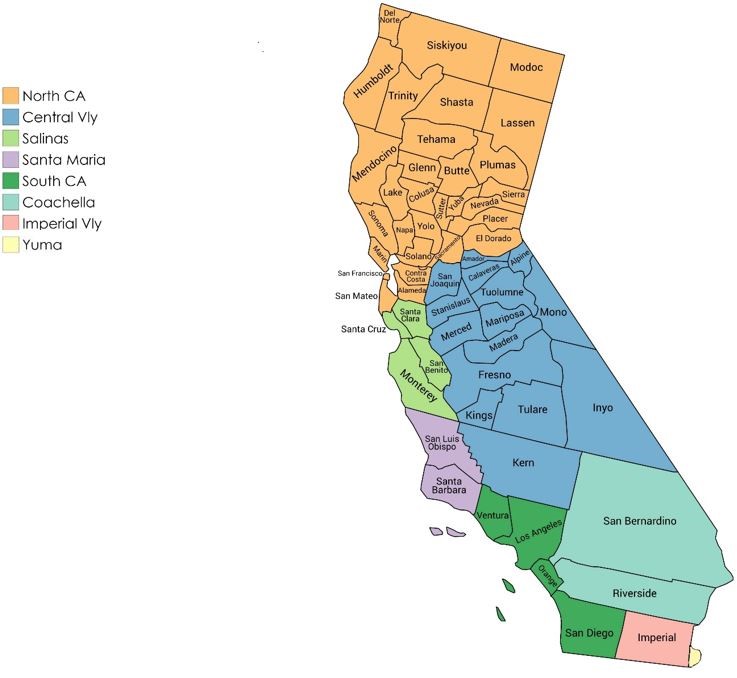
- Does a date need to be included? As long as the date of harvest can be determined using existing dates associated with the product (e.g., the "Best if used by" date or Julian date), the date of harvest does not need to be separately included alongside the growing region. Including an additional "harvest date" on consumer level packaging could be confusing and is discouraged.
- What kinds of media/materials can be used to communicate growing region? The following are recommended:
a. For processed products in bags, bowls, etc.: Ink jet or otherwise print information in a way that is legible provides high contrast (see example in Appendix A)
b. For unprocessed bagged product (e.g., romaine hearts) that are field packed: stickers/labels can be applied to bags
For cases used to transport product through the supply chain: GS1-128 PTI label (as noted in Questions 7&9, and illustrated in the exampled in Appendix B), or other sticker/marking that is prominent and legible. - For dual jurisdiction facilities, will this require a label review by USDA FSIS? “FSIS will permit a statement identifying the harvest location and date, consistent with FDA’s advice, on meat or poultry products that contain romaine lettuce. FSIS will also permit statements identifying that a meat or poultry product does not contain romaine lettuce, e.g., adding a sticker to a label that states, "Does Not Contain Romaine Lettuce." The addition of these statements to labels does not require submitting the labels to FSIS for sketch or temporary approval. Both types of statements are not considered special claims and are generically approved under Title 9 of the Code of Federal Regulations (CFR), Part 412.2.”
- What fonts and print sizes, color can be used? The information should be prominent, conspicuous and easy to read.
a. For consumer packages: While color is not specified, information is most easily read when there is visual contrast (e.g., contrast the contents or packaging graphics), upper and lower-case letters (not all upper case), and a font size no smaller than 8-point font. Different font styles are more legible than others, especially at the smaller font size. For example, OCRB is a font style that some industry members prefer.
b. For case labeling: Follow the best practices for formatting case labels with a PTI label or other comparable marking that is easy to identify and read. - Where should the information conveying region appear on the package or box?
a. For consumer packages: The information should be prominently displayed on the front (principle display panel) of a consumer package. It is recommended that this information be located close to the use by/best by date. Be aware of proper customer readability with adequate visual contract between the ink color relative to contents or packaging graphics. If stickers are used on packages that have a perforated tear off, the sticker should be applied to the part of the packaging below the tear off, so that the information is not discarded.
b. For consumer packages, information on growing regions should not appear close to the legally-required corporate address for the distributor/brand. This practice was reported as the main source of confusion during the 2019 advisory.
c. For case labeling: Follow the best practices for case labeling if using a PTI label, as explained. - Under what circumstances is an in-store point of sale sign needed for bulk, unpackaged romaine, and what should it say? Because retail and foodservice establishments promptly act on advisories, product under advisory should not be available for sale. Therefore, information on growing region does not need to be displayed by retailers on an ongoing basis. When there is an advisory, retailers should have a sign indicating that bulk, unpackaged romaine for sale is from an area not subject to the advisory.
- If products are transformed in-store and don’t bear a label what is suggested? CDC advised that “restaurants and retailers should check the label on bags or boxes of romaine lettuce, or ask their suppliers about the source of their romaine lettuce.” This is facilitated by including harvest region as part of the PTI or other case label, as noted above. Retailers transforming product in-store (e.g., prepared foods) should ensure that the romaine is not product under an advisory, and should be prepared to address consumer questions.
- Will restaurants need to provide growing region information to consumers? Foodservice operations may field consumer questions as well as adhere to potential removal advisories. Foodservice products should include regional information on the end unit or box.
- What if I ship to Canada? CFIA is not requiring any unique information for romaine at this time. If you have any questions, please contact the CFIA National Import Service Centre (NISC) from 7:00 a.m. to 3:00 a.m. (Eastern Time) at 1-800-835-4486 (Canada or USA).
- Must all points in the supply chain capture growing region information as part of their traceability information? The label/sticker information is in addition to traceability information that should be captured electronically. The Key Data Elements (e.g., the information encoded in the PTI bar code) of a robust traceability system would provide more detailed information that what is provided through this initiative
- Should the growing region be printed on the Bill of Lading?This is not practical for many companies, and is not truly traceability information. In the event of a traceback situation, this information could be useful in more quickly identifying and excluding shipments and regions that may or may not be involved in an outbreak or an advisory. However, this should not be in lieu of more granular traceability information.
Appendix A.
Example label on a bagged salad (in the upper right corner of the package; font size for the romaine declaration is font size 8, using the OCRB font style): Appendix B.
Appendix B.
Example 1. Harmonized PTI label (this example is only applicable to products containing only romaine, not blends).

Example 2. Alternative format of human readable information, while maintaining the format of the PTI barcode.

